- AFRICA CENTRE OF EXCELLENCE FOR NEGLECTED TROPICAL DISEASES AND FORENSIC BIOTECHNOLOGY

The research on Trypanosomiasis is multifaceted, with primary objectives focused on establishing a region-wide map of Trypanosoma species distribution in mammalian hosts and tsetse vectors. The unit employs optimized trypanosomal infection detection methods for comprehensive mapping. Additionally, molecular identification and characterization of Trypanosoma parasites constitute a significant aspect of the research.
An innovative facet of this initiative involves the exploration of DNA-based vaccines utilizing molecular targets identified through novel approaches in molecular biology. The research unit is committed to studying vaccine-guided delivery systems, employing recombinant DNA, bionanoparticles, or a combination thereof. This holistic approach aims to contribute not only to understanding the geographical spread of trypanosomiasis but also to developing effective interventions based on advanced molecular techniques.
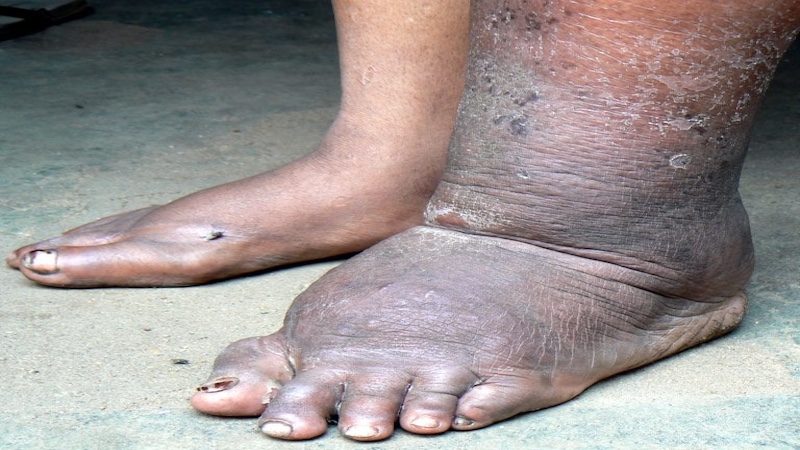
The Africa Center for Excellence research unit on Filiariasis is dedicated to researching the diagnosis and distribution of Filaria parasites in Nigeria and the sub-region. Beyond mapping, the unit delves into the study of vectors, including Anopheles gambiae, A. funestus, and Culex quinquefasciatus, involved in transmission dynamics. Given the interconnectedness of neglected tropical diseases (NTDs), vaccination experiments with other NTDs, such as trypanosomiasis and rabies, will be conducted to investigate potential interactions and optimize novel DNA- and/or bionanoparticle-based vaccination strategies.
This research initiative embodies a comprehensive approach to understand filariasis epidemiology, vectors, and potential synergies with other NTDs, ultimately contributing to the development of integrated and effective control measures.

The research unit dedicated to Rabies focuses on deploying advanced molecular biotechnologies for a more sensitive and specific molecular diagnosis of rabies in dogs and other animals. The unit seeks to determine the circulating genotypes in Nigeria and the sub-region, forming the foundation for subsequent research endeavors.
An integral objective is building on existing groundwork for the development of a recombinant DNA vaccine for rabies. This entails creating a vaccine anchored on knowledge of circulating genotypes that is safe, stable, and effective, addressing challenges associated with existing vaccines. Concurrent field-based studies on the ecology of dogs aim to guide proposed intervention strategies, recognizing that controlling this fatal disease necessitates comprehensive measures in the principal reservoir. The research unit thus combines molecular diagnostics, vaccine development, and ecological studies for a holistic approach to rabies control.
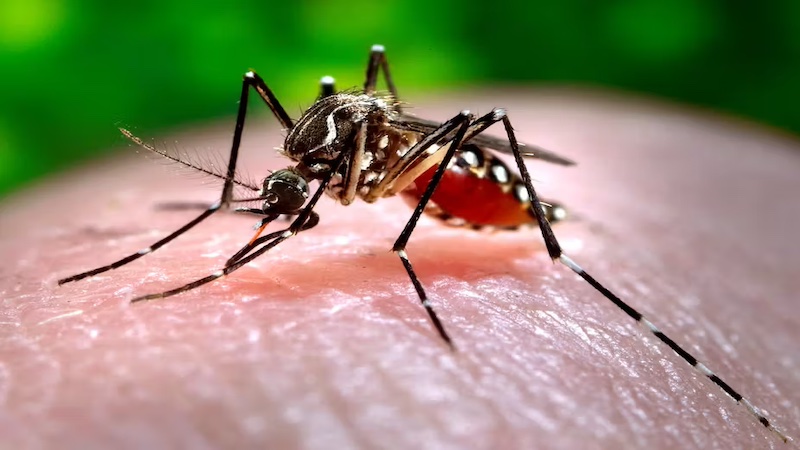
The Dengue research initiative seeks to comprehensively investigate the epidemiology and transmission dynamics of the dengue virus within the targeted region. A primary goal is to create a detailed map of the distribution of Aedes mosquitoes, the main vectors responsible for dengue transmission, identifying high-risk areas. The research will employ state-of-the-art diagnostic tools to facilitate early detection of dengue infections. Genetic diversity studies of circulating dengue virus strains will inform the development of potential vaccines and antiviral strategies.
Furthermore, the research unit will explore innovative approaches, such as leveraging bionanoparticles and molecular biology techniques, to enhance the efficacy of vaccine delivery. This multi-faceted research aims to contribute significantly to the understanding of dengue transmission, leading to the development of effective control measures.
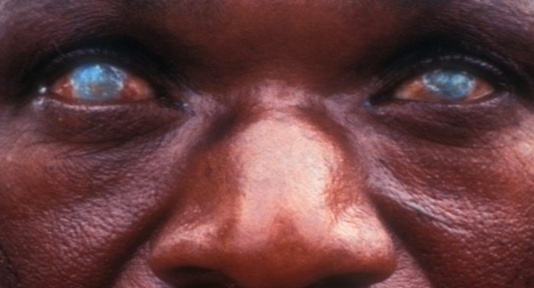
The Onchocerciasis research unit aims to comprehensively understand the distribution and prevalence of Onchocerca volvulus, the causative agent of onchocerciasis, in both human and Simulium vectors within the target region. Molecular identification techniques will be employed to characterize different strains of the parasite. Concurrently, the research will investigate the ecology and behavior of Simulium species to enhance understanding of transmission dynamics.
Emphasis will be placed on developing novel DNA-based interventions, including vaccines, targeting specific molecular markers crucial in interrupting the life cycle of the parasite. The research unit will explore recombinant DNA and bionanoparticles for potential advancements in vaccine development and delivery. This comprehensive research is poised to make significant contributions to the control and prevention of onchocerciasis.
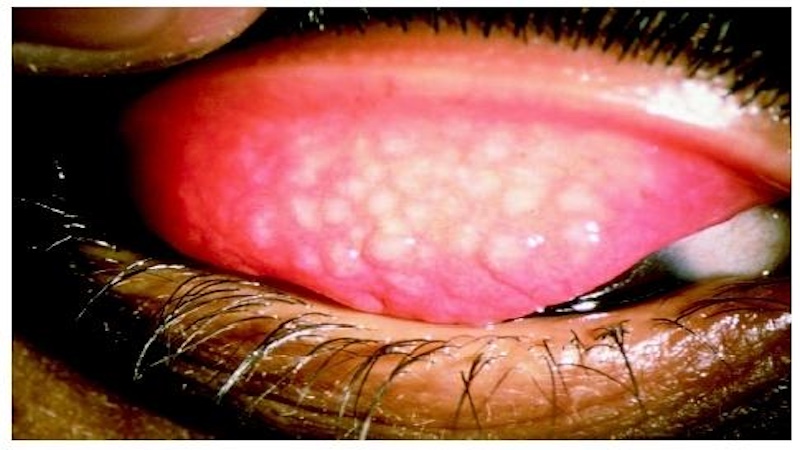
The Trachoma research unit focuses on understanding the epidemiology and prevalence of Chlamydia trachomatis, the bacterium responsible for trachoma, in the designated region. Efforts will be directed towards mapping the distribution of the disease and its vectors, particularly Musca sorbens. Molecular identification methods will be employed for strain characterization and differentiation.
The research will explore innovative approaches for the development of DNA-based vaccines, leveraging molecular targets essential in disrupting the transmission cycle. Additionally, studies will focus on implementing control strategies based on comprehensive field-based investigations and ecological studies to guide intervention measures effectively. This holistic approach aims to address the intricate dynamics of trachoma and contribute to its effective control.
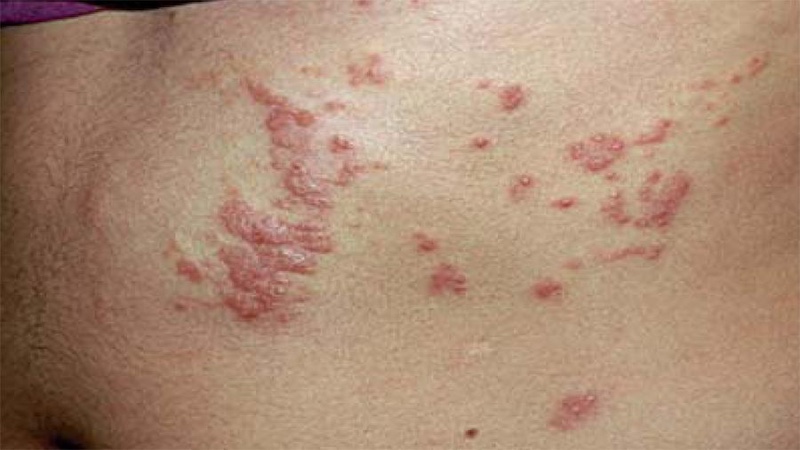
Research on Schistosomiasis aims to create a comprehensive understanding of the distribution and prevalence of Schistosoma species in the region, focusing on both human and snail hosts. Molecular identification techniques will be employed to characterize different Schistosoma strains. The research will investigate the ecology of snail vectors, emphasizing their role in transmission dynamics.
Furthermore, the unit will explore DNA-based vaccines, with a focus on disrupting the life cycle of the parasite. Innovative methods such as recombinant DNA and bionanoparticles will be investigated for vaccine delivery, aiming at effective control and prevention strategies against schistosomiasis. This research endeavors to provide a robust foundation for the development of targeted interventions against schistosomiasis.
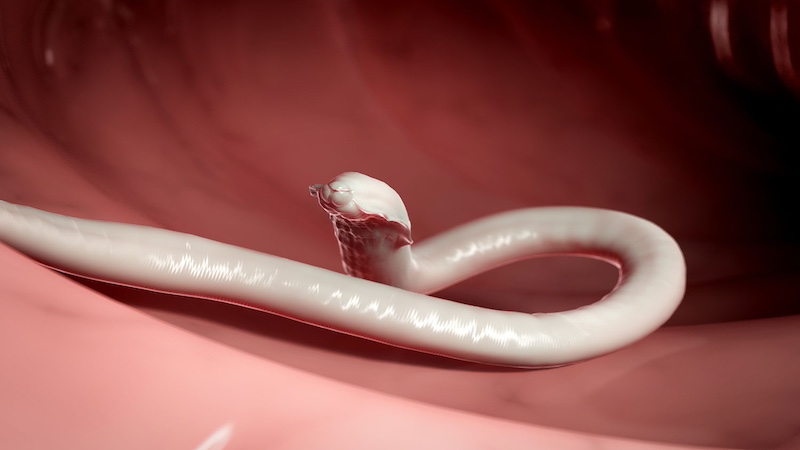
The research unit on Soil Transmitted Helminthiasis aims to establish a comprehensive understanding of the distribution and prevalence of helminth infections in the soil, focusing on key species. Molecular identification methods will be employed to characterize different helminth strains and investigate their transmission dynamics.
The research will also explore the ecology of the soil environment and its impact on helminth survival and transmission. DNA-based vaccines will be a key focus, with an emphasis on disrupting the life cycle of helminths. Innovative delivery approaches, including recombinant DNA and bionanoparticles, will be investigated to enhance vaccine efficacy and contribute to the development of effective control strategies against soil-transmitted helminthiasis. This research initiative aspires to make substantial contributions to the understanding and management of soil-transmitted helminthiasis.